Which of the Following Compounds Is Not Aromatic
Aromatic compound- The term aromaticity is used to describe a cyclic planar molecule with a ring of resonance bonds that exhibits more stability than other geometric or connective arrangements with the same set of atoms. Which of the following isare aromatic compounds.

Aromatic Compounds Organic Chemistry Organic Chemistry Study Chemistry Lessons
Its interacted by two sp 3 carbons.

. Every atom in the ring must be able to participate in resonance. First week only 499. The compound must be monocyclic.
The easiest way to put it is this. So the correct answer is Option D. Why is the following compound not aromatic.
Another question on Chemistry. This compound is not aromatic. Hence option D is not an aromatic compound because it does not obeys.
So the compound is aromatic. The compound must have a conjugated system with a p orbital at every vertex D. In A 2π electrons obeys the 4n2π rule cyclic and conjugated so aromatic.
It has 4n2 pi electrons. So it is an aromatic compound. It is not cyclic.
2They are conjugated all around the ring. B is anti-aromatic with 4 πe. The p electron system is not continuous.
The compound must satisfy Hückels rule -must have 4n 2 electrons. CH3-CH-CHCH2 In the IUPAC name for the following compound the -Br group is located at position Br Select one. The correct rate of reaction of given compounds towards electrophilic aromatic substitution reaction is.
B Not planar Hence it is non-aromatic. Asked Apr 16 2019 in Chemistry by AmreshRoy 695k points jee mains 2019 1 vote. Welcome to Sarthaks eConnect.
The p electron system is not continuous. Which of the following is not an aromatic compound. I Aromatic homocyclic compound ii Aromatic heterocyclic compound All heterocyclic compounds need not be aromatic.
It has 4n2 electrons. Huckels rule as it has conjugated 4 -electron system. It is not cyclic.
Name the following aromatic compound. Five is an odd number. CH-CH2 CH3 CH3-CH2 b.
It has 4n electrons. Benzenoids are the compounds having at least one benzene ring. D is non aromatic because of sp3 carbon in the ring.
Which of the following compounds is not considered aromatic. The correct option is B B C and D. And we can see there is a congregation enter.
Give two examples for each of the following type of organic compounds. In C 8π electrons does not obey the 4n2π rule so anti-aromatic. Which of the following isare aromatic compounds.
Which of the following compounds is not considered to be aromatic. Unlike aromatic compounds which follow Hückels rule 4 n 2 π electrons and are highly stable anti-aromatic compounds are highly unstable and highly reactive. What is the empirical formula of this compound.
2 0 03 O d4 Which of the following compounds is trans-3-hexene. Which one of the following compounds is non-aromatic. 4The molecule must be flat.
N H N None of these are aromatic. Solution for Which of the following compounds is the most reactive in the nucleophilic aromatic substitution reaction with NaOH. When 1130 g of the compound is burned in oxygen 1064 g co2 and 03631 g h2o are produced.
Weve got the study and writing resources you need for your assignments. Why is the following compound not aromatic. Which of the compounds is are aromatic s.
Aromatic compounds are categorized further into two categories ie. Aromatic molecules have the following characteristics. The compound must be cyclic and planar B.
All of these are aromatic. There are five compares in congregation one from the nine and one from each double bar. N H N None of these are aromatic.
3the molecule must have 4n2 Pi electrons. C is anti-aromatic with 8 πe. Please log in or register to add a comment.
CH3 H CH3-CH2 H ос H CH2-CH3. Acompound contains c h and o atoms. Start your trial now.
A unique platform where students can interact with teachersexperts. Among the following four aromatic compounds which one will have the lowest melting point. Prev Question Next Question.
All of these are aromatic. Which of the following structures violates the octet rule. It has 4n pi electrons.
Solve any question of Hydrocarbons with-. Lawner tetra New nine first when you destroyed. Which one of the following statements is not true for a compound to be considered as aromatic.
This compound is not sacred. Rest of the species are aromatic at each of them belongs to 6 -electron 4n 2π n 1 system. The benzene ring as a branch is called a _____ group.
In B 4π electrons does not obey the 4n2π rule so anti-aromatic. In D system is not conjugated so non-aromatic. In the last we can conclude that phenol naphthalene and pyridine are aromatic compounds.
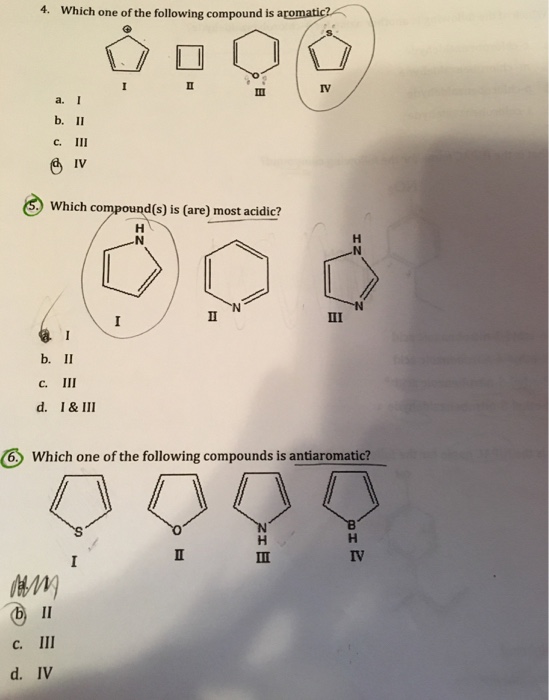
Solved 4 Which One Of The Following Compound Is Aromatic Chegg Com

Aromatic Compounds Organic Chemistry Notes Chemistry Chemistry Notes

Aromaticity Practice Quiz Chemistry Lessons Organic Chemistry How To Memorize Things

Classification Of Aromatic Antiaromatic And Nonaromatic Compounds Chemistry Lessons Organic Chemistry Study Chemistry

Among The Above The Number Of Aromatic Compound S Is Are

Newman Projections Video Tutorials By Leah4sci Organic Chemistry Organic Chemistry Study Chemistry Basics

Organic Chemistry I Study Guides Organic Chemistry Chemistry Chemistry Basics

Aromatic Compounds Study Guide Cheat Sheet Organic Chemistry Study Chemistry Help Teaching Chemistry

Aromatic Compounds Are Highly Stable Due To Resonance Effect Benzene Benzene Derivatives Aromatic

Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps

Antiaromaticity And Antiaromatic Compounds Master Organic Chemistry Chemistry Chemistry Notes Organic Chemistry

Naphtalene 10 Annulene Represent An Aromatic And Nonaromatic Compounds Chemistry Aromatic Molecules

Complete Collection Organic Chemistry Study Organic Chemistry Notes Organic Chemistry

Aromatic Antiaromatic Or Nonaromatic Practice Session 2 Youtube

Phenol Is An Aromatic Organic Compound With The Molecular Formula C6h5oh It Is A White Crystalline Solid That Is Volatil Chemistry Notes Chemistry Molecular

Aromaticity Mcqs For Neet With Answers

A Reaction Map Pdf For Benzene And Aromatic Compounds Organic Chemistry Books Organic Chemistry Reactions Organic Chemistry


Comments
Post a Comment